A Philadelphia tech company has suffered a severe ransomware attack. The incident is specially warning as the affected company provides the software currently used in COVID-19 vaccine investigation. The global firm eResearchTechnology (ERT) first came under attack last September 20.
According to the report, one of the company’s employees discovered that they were locked out of their data by a ransomware variant; although clinical trials were not at risk, the company mentioned that some of its regular tasks were carried out with pen and paper due to the disruption.
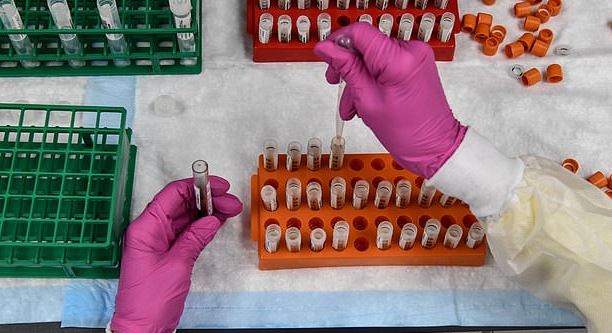
Among the affected ERT partners is IQVIA, the contract research organization helping manage AstraZeneca’s COVID vaccine trial, and Bristol Myers Squibb, a world leading drugmaker consortium. ERT has not said how many clinical trials were affected, but its software is being used in drug trials across Europe, Asia and North America.
Drew Bustos, ERT’s directive, said the company took its systems offline on September 20 as a security measure and asked cybersecurity experts to help. The Federal Bureau of Investigation (FBI) was also notified: 2Nobody feels great about these experiences, but the incident has been contained”, said Bustos.
The affected systems started working again last Friday, as full recovery is expected to be accomplished this week. The company’s directives refused to disclose further details such as the ransomware variant employed by the attackers or the ransom amount demanded.
On the other hand, IQVIA issued a statement saying that the attack had a limited impact on their clinical trials operations: “We are not aware of any confidential data or patient information, related to our clinical trial activities, that have been removed, compromised or stolen,” the company said.
Pfizer and Johnson & Johnson, the other two companies working on a coronavirus vaccine using the affected systems, said their current developments had not been affected.

He is a well-known expert in mobile security and malware analysis. He studied Computer Science at NYU and started working as a cyber security analyst in 2003. He is actively working as an anti-malware expert. He also worked for security companies like Kaspersky Lab. His everyday job includes researching about new malware and cyber security incidents. Also he has deep level of knowledge in mobile security and mobile vulnerabilities.